Complex biological molecules consist of Carbon atoms bonded with other elements, especially oxygen and hydrogen and frequently also nitrogen, phosphorus, and sulfur (collectively known as CHNOPS).
Carbon is capable of forming a vast number of compounds, more than any other element, with almost ten million compounds described to date.
The most important characteristics of Carbon as a basis for the chemistry of life are that each Carbon atom is capable of forming up to four valence bonds with other atoms simultaneously, and that the energy required to make or break a bond with a Carbon atom is at an appropriate level for building large and complex molecules which may be both stable and reactive.
We live because of Carbon.
Wood, coal, petroleum, coke, diesel, gasoline, kerosene, LPG and other fuels consist of Carbon.
The heat energy released by reactions of fuels can be used for warmth, cooking, or industrial processes or can be converted into mechanical energy via a heat engine.
From camp fire to Stirling, Diesel and Otto engines, from cooking food to producing metals – all these processes demand carbon-based fuels.
Our human civilization is powered by Carbon.
Steel is an alloy made up of iron with typically a few tenths of a percent of Carbon to improve its strength.
Diamond is a solid form of the element Carbon, which is used in jewellery and industry.
Graphite is a crystalline form of the element Carbon, which is used for making lubricants, carbon brushes, refractories, drilling muds, carburizing iron, manufacturing synthetic diamonds, electrodes, batteries, and solar panels.
Amorphous carbon is free, reactive Carbon that has no crystalline structure.
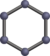
Fullerene is an allotrope of Carbon whose molecule consists of Carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh.
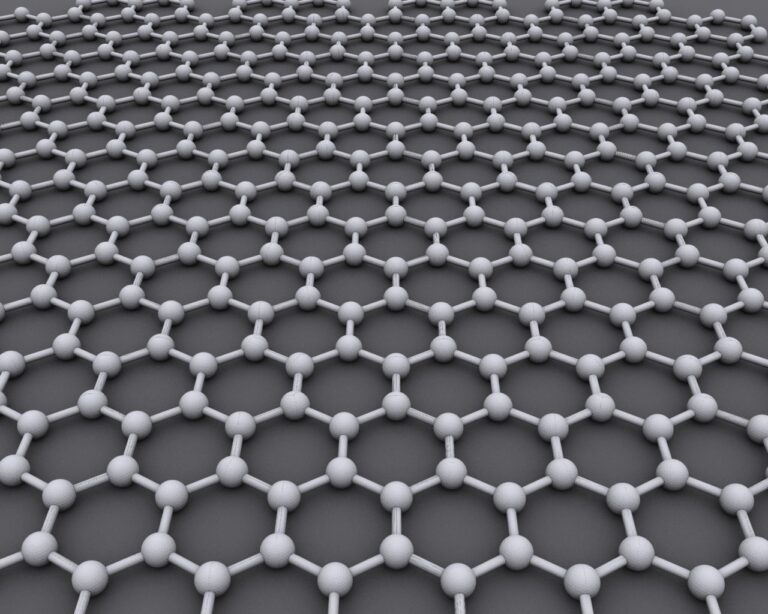
Graphene is an allotrope of Carbon consisting of a single layer of atoms arranged in a two-dimensional honeycomb lattice nanostructure.
Our human civilization develops on Carbon-based materials.
Carbon, in conclusion
Carbon is the 15th most abundant element in the Earth’s crust, and the fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen.
The symbol C and atomic number 6.
It is nonmetallic and tetravalent — making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table.
Carbon makes up only about 0.025 percent of Earth’s crust, but is very important for our civilization.
Carbon’s abundance, its unique diversity of organic compounds, and its unusual ability to form polymers at the temperatures commonly encountered on Earth enables this element to serve as a common element of all known life. It is the second most abundant element in the human body by mass (about 18.5%) after oxygen.
The atoms of carbon can bond together in diverse ways, resulting in various allotropes of carbon. Well-known allotropes include graphite, diamond, amorphous carbon and fullerenes.
More compounds are known which contain Carbon than don’t.
The graphite in a typical mechanical pencil has a diameter of 0.7 mm.This is equal to 2 million layers of graphene.
Car tires are black because they are about 30% carbon black, which is added to rubber to strengthen it. The carbon black also helps to protect against UV damage to tires.
Carbon is a part of the “ash” formed by helium burning within stars (during nuclear reactions).